With just one shot, the new crown vaccine from the team of Chinese Academician Chen Wei was approved for marketing on February 25
According to the website of the State Drug Administration today, the State Drug Administration has conditionally approved the new crown vaccine registration applications of Cansino Biotech Co., Ltd. and Sinopharm Zhongsheng Wuhan Company. It is reported that the team of Academician Chen Wei of the Academy of Military Medicine of the Academy of Military Sciences and Kansino Biosciences jointly developed a recombinant new coronavirus vaccine (type 5 adenovirus vector). According to the news, the vaccine only needs one shot.
According to the website of the National Medical Products Administration, the vaccine is the first domestically-made adenovirus vector new coronavirus vaccine approved and is suitable for preventing the disease (COVID-19) caused by the new coronavirus infection.
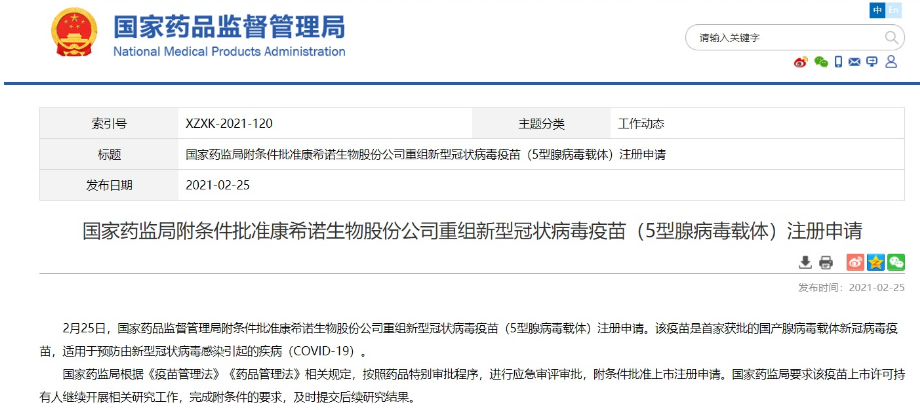
According to the relevant provisions of the "Vaccine Administration Law" and "Drug Administration Law", the State Food and Drug Administration conducts emergency review and approval in accordance with the special drug approval procedures, and approves the application for listing registration with conditions. The State Food and Drug Administration requires the vaccine marketing license holder to continue to carry out relevant research work, complete the conditional requirements, and submit follow-up research results in a timely manner.
 小任班长的博客
小任班长的博客 

